Research
Development of an Artificial Kidney
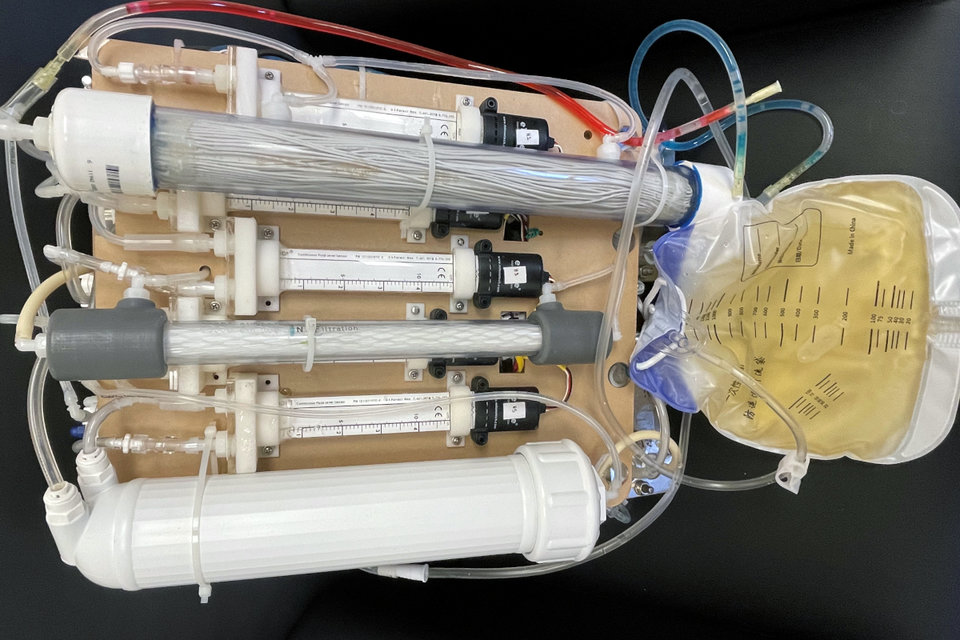
Current clinical approaches to treat patients with end stage renal disease (ESRD) include hemodialysis, peritoneal dialysis, and renal transplantation. We have developed a novel dialysis-free and waterless artificial kidney technology that has the potential to mimic the filtration properties of the renal glomerulus and the ion/water transport processes in the nephron. Importantly, the device does not utilize external water/dialysate or living cells. This waterless technology creates more than a replacement for dialysis, it allows increased freedom for patients. Since the technology does not utilize dialysate, the portable device we are developing is self-enclosed and operated without having to worry about where to source or dispose of dialysate and water. Additionally, for providers, our waterless technology will eliminate the massive water infrastructure and associated costs currently needed to perform standard dialysis treatments.
Structural Biology of Membrane Transport Proteins
Understanding the complexity of life requires that scientists have a deeper understanding of how molecules in cells function and interact. Integral membrane proteins play key roles in cell transport of ions and substrates, signaling and receptor function. It is estimated that approximately 25% of the human genome encodes membrane proteins. They are important targets for pharma as approximately 60% of drugs on the market target membrane proteins. Mutations in these proteins are associated with disorders such as diabetes, obesity, cancer, obesity, Alzheimer’s and Parkinson’s disease.
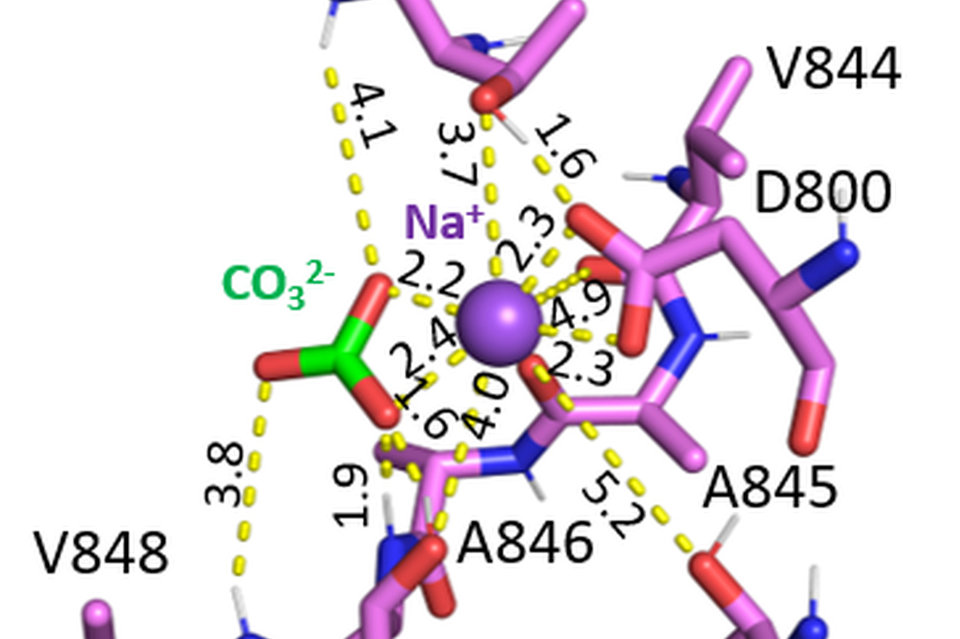
Structural biology has played a key role at the atomic level in understanding structure function relationships of membrane proteins. Techniques such as x-ray crystallography, cryo-EM, NMR and neutron diffraction have played important roles. The importance of SLC4 bicarbonate/carbonate transporters in human biology is highlighted by the diseases associated with their functional loss including blindness, short stature, abnormal cognitive function, cerebral calcification, metabolic acidosis, anemia and hearing abnormalities. By combining cryo-EM structural analysis with functional mutagenesis studies, we have reported for the first time the near-atomic structures of the electrogenic NBCe1, an electrogenic Na+-CO32- cotransporter and NDCBE, the Na+-CO32-/Cl-exchanger.
Functional Properties of SLC4 Transporters
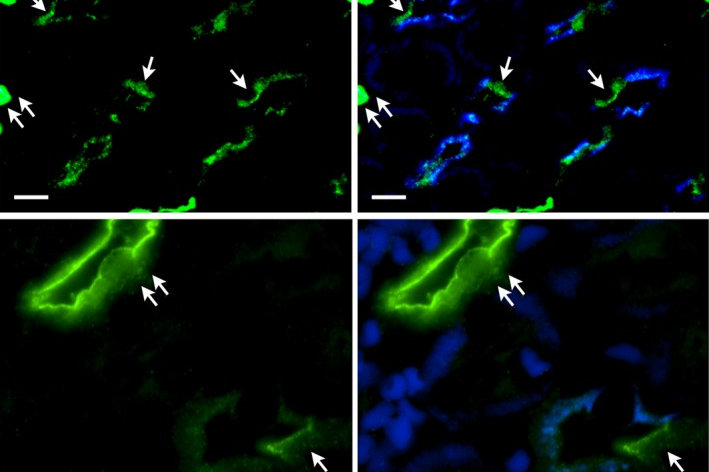
SLC4 transporters are a family 10 membrane proteins most of whom transport bicarbonate or carbonate coupled to Na+ and/or Cl- in many cell types. These proteins play key roles in the brain, heart, kidney, eye and ear and are involved in the maintenance of systemic acid-base chemistry and in maintaining the normal ionic milieu of various cell types. Our laboratory has discovered and cloned several SLC4 transporters and has characterized their functional properties using microscopic fluorescent pH and patch clamp methodologies. One of the transporters, SLC4A11 which is involved in ocular CHED disease has been a recent focus of the lab where we have published evidence that this transporter is unique among SLC4 proteins in that it transports NH3and a H+. The role of SLC4A11 in the kidney is not well understood. We demonstrated the selective expression of SLC4A11 in the nephron upper descending thin limbs (DTLs) (which are aquaporin (AQP1)‐positive) in the outer medulla and inner medulla with little or no expression in the lower DTLs (which are AQP‐1‐null). SLC4A11 also colocalized with AQP1 and the urea transporter UT‐B in the mouse descending vasa recta but was absent in mouse and rat ascending vasa recta. Mouse, but not rat, outer medullary collecting duct cells also labeled for SLC4A11. We hypothesized that in the inner stripe of the outer medulla, SLC4A11 plays a role in the countercurrent transport of ammonia absorbed from the outer medullary thick ascending limb and secreted into the long‐looped DTLs.
Artificial Intelligence and Nephrology
In recent years, significant breakthroughs have been made in the field of natural language processing, particularly with the development of large language models (LLMs). LLMs have demonstrated remarkable capabilities on benchmarks related to general medical question answering, but there is less data about their performance in subspecialty fields and fewer studies still comparing the many available LLMs. These models have the potential to be used as a part of adaptive physician training, medical copilot applications, and digital patient interaction scenarios. We recently investigated the medical knowledge capability of multiple LLMs in the context of their internal medicine subspecialty multiple-choice test-taking ability. We compared the performance of several open-source LLMs (Llama2-70B, Koala 7B, Falcon 7B, Stable-Vicuna 13B, and Orca-Mini 13B) with the proprietary models GPT-4 and Claude 2 on multiple-choice questions in the field of nephrology. We showed that the current widely used open-source LLMs have poor zero-shot reasoning ability in nephrology compared with GPT-4 and Claude 2, illustrating knowledge gaps across LLMs relevant to future subspecialty medical training and patient care.
We are also utilizing AI approaches to optimize decision-making in patients who receive CRRT. This dialytic approach is utilized in patients with suboptimal hemodynamics and is associated with a high mortality. There is currently insufficient data and clinical criteria to determine which patients should be started on CRRT and which patients should have CRRT stopped. We have recently developed novel AI models to predict which patients should be treated with CRRT that can be tested in future clinical trials to determine their utility clinically.