Research
Structural Biology of Membrane Transport Proteins
Understanding the complexity of life requires that scientists have a deeper understanding of how molecules in cells function and interact. Integral membrane proteins play key roles in cell transport of ions and substrates, signaling and receptor function. It is estimated that approximately 25% of the human genome encodes membrane proteins. They are important targets for pharma as approximately 60% of drugs on the market target membrane proteins. Mutations in these proteins are associated with disorders such as diabetes, obesity, cancer, obesity, Alzheimer’s and Parkinson’s disease.
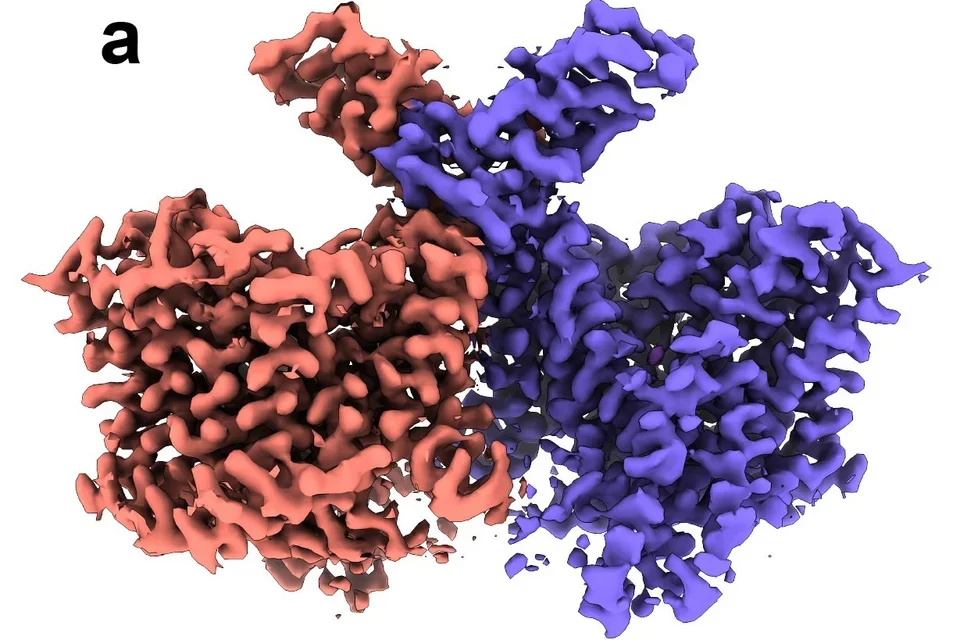
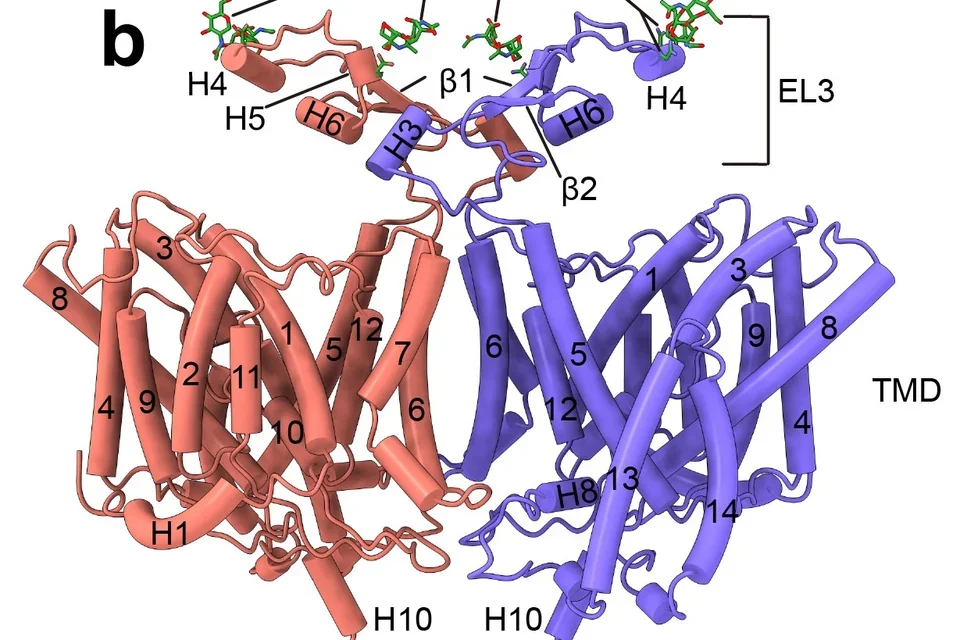
Structural biology has played a key role at the atomic level in understanding structure function relationships of membrane proteins. Techniques such as x-ray crystallography, cryo-EM, NMR and neutron diffraction have played important roles. The importance of SLC4 bicarbonate/carbonate transporters in human biology is highlighted by the diseases associated with their functional loss including blindness, short stature, abnormal cognitive function, cerebral calcification, metabolic acidosis, anemia and hearing abnormalities. By combining cryo-EM structural analysis, computational modeling and functional mutagenesis studies, we have reported for the first time the near-atomic structures of NBCe1, NDCBE, and conformational changes in AE1 during its transport cycle.
NBCn1: A Structural Mechanism That Breast Tumors Exploit to Survive Acid Stress
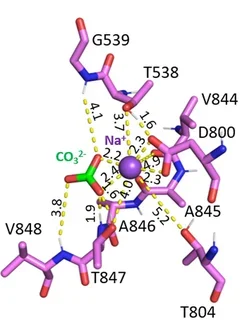
Breast cancer cells exist in a harsh, acidic microenvironment created by elevated glycolytic metabolism, yet they maintain an alkaline intracellular pH that promotes proliferation, metastasis, and drug resistance. The sodium-coupled base transporter NBCn1 (SLC4A7) plays a central role in enabling this reversal of the pH gradient. Using cryo-EM together with extensive molecular dynamics and free-energy modeling, we defined the transporter’s outward-facing, inward-facing, and occluded intermediate states. We discovered that NBCn1 operates through a highly efficient elevator-type transport cycle with minimal structural rearrangement and an exceptionally high turnover rate (~15,000 s⁻¹). These properties allow breast cancer cells to import base equivalents at rates unmatched by other transporters, sustaining intracellular alkalinity even under severe extracellular acidosis. Importantly, our structural analysis reveals that NBCn1 transports 2Na⁺–CO₃²⁻, providing a thermodynamic advantage for base loading across a wide range of tumor pH conditions. Together, these findings identify NBCn1 as a promising therapeutic target for disrupting pH homeostasis in breast tumors.
Artificial Intelligence and Nephrology
In recent years, large language models (LLMs) have achieved impressive performance on general medical question-answering benchmarks, yet far less is known about their capabilities in subspecialty domains. We evaluated multiple LLMs including open-source models such as Llama2-70B, Koala 7B, Falcon 7B, Stable-Vicuna 13B, and Orca-Mini 13B against proprietary models like GPT-4 and Claude 2 on nephrology multiple-choice examinations. Our results showed that widely used open-source LLMs perform poorly in zero-shot nephrology reasoning compared with GPT-4 and Claude 2, highlighting substantial knowledge gaps relevant to future subspecialty training and clinical decision support. Recent follow-up studies extended this work by examining retrieval-augmented generation (RAG) in nephrology: surprisingly, even adversarial databases can improve test-taking performance in certain models, revealing that attention-shifting rather than domain knowledge may drive some RAG benefits. In a separate study, we introduced AutoMedPrompt, a textual-gradient optimization method that systematically improves system prompts for medical reasoning. AutoMedPrompt enabled Llama-3 to surpass GPT-4, Claude 3 Opus, and Med-PaLM 2 on several medical benchmarks including NephSAP without any model fine-tuning, demonstrating that optimized prompting alone can elevate open-source models to competitive clinical performance levels.
We are also utilizing AI approaches to optimize decision-making in patients who receive CRRT. This dialytic approach is utilized in patients with suboptimal hemodynamics and is associated with a high mortality. There is currently insufficient data and clinical criteria to determine which patients should be started on CRRT and which patients should have CRRT stopped. We have recently developed novel AI models to predict which patients should be treated with CRRT that can be tested in future clinical trials to determine their utility clinically.
Development of an Artificial Kidney
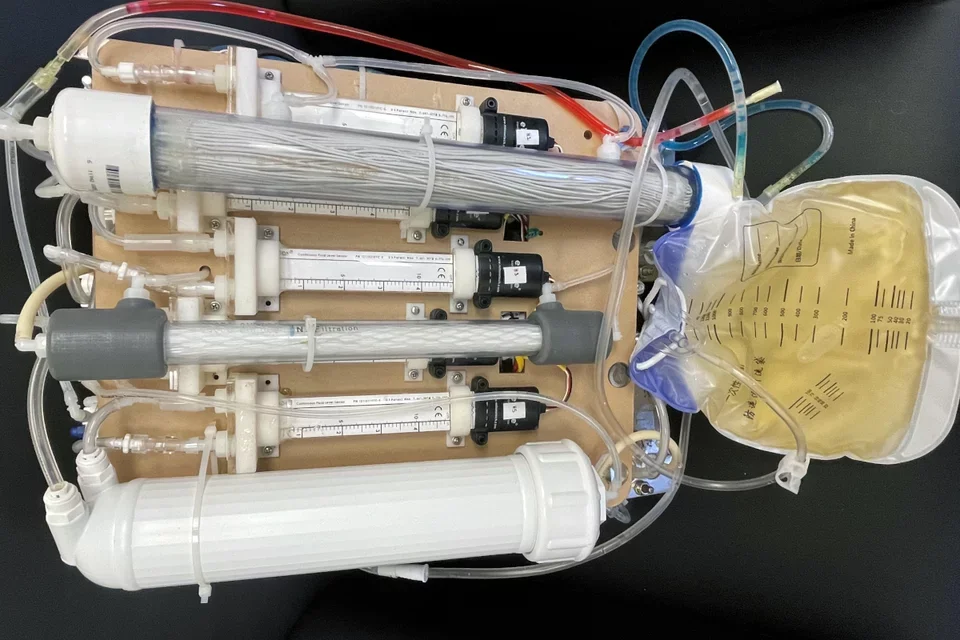
Current clinical approaches to treat patients with end stage renal disease (ESRD) include hemodialysis, peritoneal dialysis, and renal transplantation. We have developed a novel dialysis-free and waterless artificial kidney technology that has the potential to mimic the filtration properties of the renal glomerulus and the ion/water transport processes in the nephron. Importantly, the device does not utilize external water/dialysate or living cells. This waterless technology creates more than a replacement for dialysis, it allows increased freedom for patients. Since the technology does not utilize dialysate, the portable device we are developing is self-enclosed and operated without having to worry about where to source or dispose of dialysate and water. Additionally, for providers, our waterless technology will eliminate the massive water infrastructure and associated costs currently needed to perform standard dialysis treatments.
Biocompatibility and the ability to mediate the appropriate flux of ions, urea, and uremic toxins between blood and dialysate components are key parameters for membranes used in dialysis. Oxone-mediated TEMPO-oxidized cellulose nanomaterials have been demonstrated to be excellent additives in the production and tunability of ultrafiltration and dialysis membranes. We have developed a novel nanocellulose ionic liquid membrane (NC-ILM) that was tested in vitro and ex vivo. An increase in flux of up to two orders of magnitude was observed with increased rejection (about 99.6%) of key proteins compared to that of polysulfone (PSf) and other commercial membranes. NC-ILMs have a sharper molecular weight cut-off than other phase inversion polymeric membranes, allowing for high throughput of urea and a uremic toxin surrogate and limited passage of proteins in dialysis applications. Superior anti-fouling properties were also observed for the NC-ILMs, including a > 5-h operation time with no systemic anticoagulation in blood samples. Finally, NC-ILMs were found to be biocompatible in rat ultrafiltration and dialysis experiments, indicating their potential clinical utility in dialysis and other blood filtration applications.